MFDA Bans Cofsy Cough Syrup Over Quality Concerns
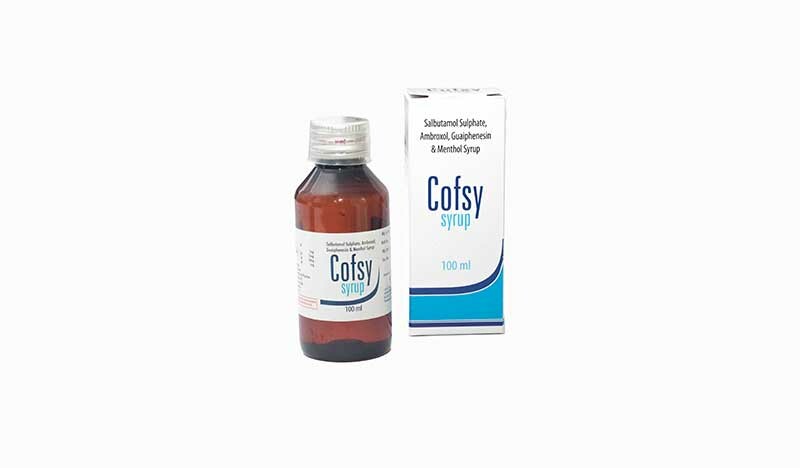
The Maldives Food and Drug Authority (MFDA) has announced a ban on Cofsy, a widely used cough syrup manufactured in India, due to concerns over its quality.
In a statement released today, the MFDA revealed that the decision was prompted by the discovery of “particles” in the syrup. The authority has indicated that it is conducting further tests to evaluate the safety of the product.
https://x.com/MFDA_mv/status/1825437145005469927
“Consequently, the import, sale, and consumption of this medicine are prohibited until the MFDA completes its tests and provides further information,” the statement said.
For additional details, the public is advised to contact the MFDA’s hotline at 7200321.
Cofsy syrup, produced by MMC Healthcare Ltd of India, is a combination medication used to treat coughs with mucus and alleviate symptoms such as a runny nose, sneezing, itching, and watery eyes.