MFDA Issues Caution Against Panadol Usage Pending Testing Results

The Maldives Food and Drug Authority (MFDA) has issued a directive halting the consumption of Panadol, a widely used over-the-counter pain reliever, following concerns over its quality.
In a statement released today, the MFDA revealed that it had identified potential quality issues with the medication during recent qualitative and quantitative assessments conducted on random samples sourced from the market.
During these tests, certain tablets were found to exhibit defects such as chipping and cracking, which the agency attributed to possible lapses in adhering to appropriate manufacturing and storage standards.
The MFDA expressed apprehension that such compromised tablets may not deliver the expected therapeutic effects.
Additional investigations are underway to ascertain the underlying cause of these defects, the agency confirmed.
The MFDA further mandated the segregation of existing stocks of the medication within pharmacies, warehouses, and stores, emphasizing the importance of proper storage conditions.
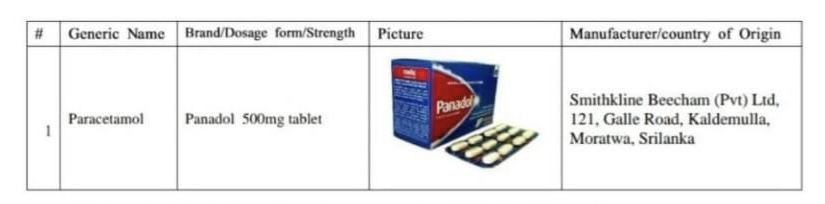
Panadol, manufactured by Sri Lanka’s Smithkline Beecham, stands as one of the most prevalent pain relief options utilized in the Maldives.